The Future of Men’s Healthcare: A Path to Improved Access, Outcomes, and Cost Savings
Telehealth empowers men to take control of their health in a way that is easy, confidential, and fits into their busy lifestyles.

Telehealth empowers men to take control of their health in a way that is easy, confidential, and fits into their busy lifestyles.
Contraline released promising data from the clinical trial it’s conducting to test the efficacy of Adam, its male birth control product. The nonhormonal gel seems to be doing a good job of blocking the flow of sperm to the vas deferens, and no serious adverse events have been reported.

Bright Uro raised $23 million in Series A funds to help achieve FDA clearance for its urodynamics system and launch the product in the U.S. Should it be cleared, the system will become the first product on the market able perform urodynamic monitoring wirelessly and without a catheter, the company's CEO said.
Avenda Health, a startup focused on personalized prostate cancer care, recently received 510(k) clearance from the FDA for its cancer mapping platform. The platform uses a patient’s own diagnostic data to define the extent of the disease and create a cancer probability map with optimal treatment margins.
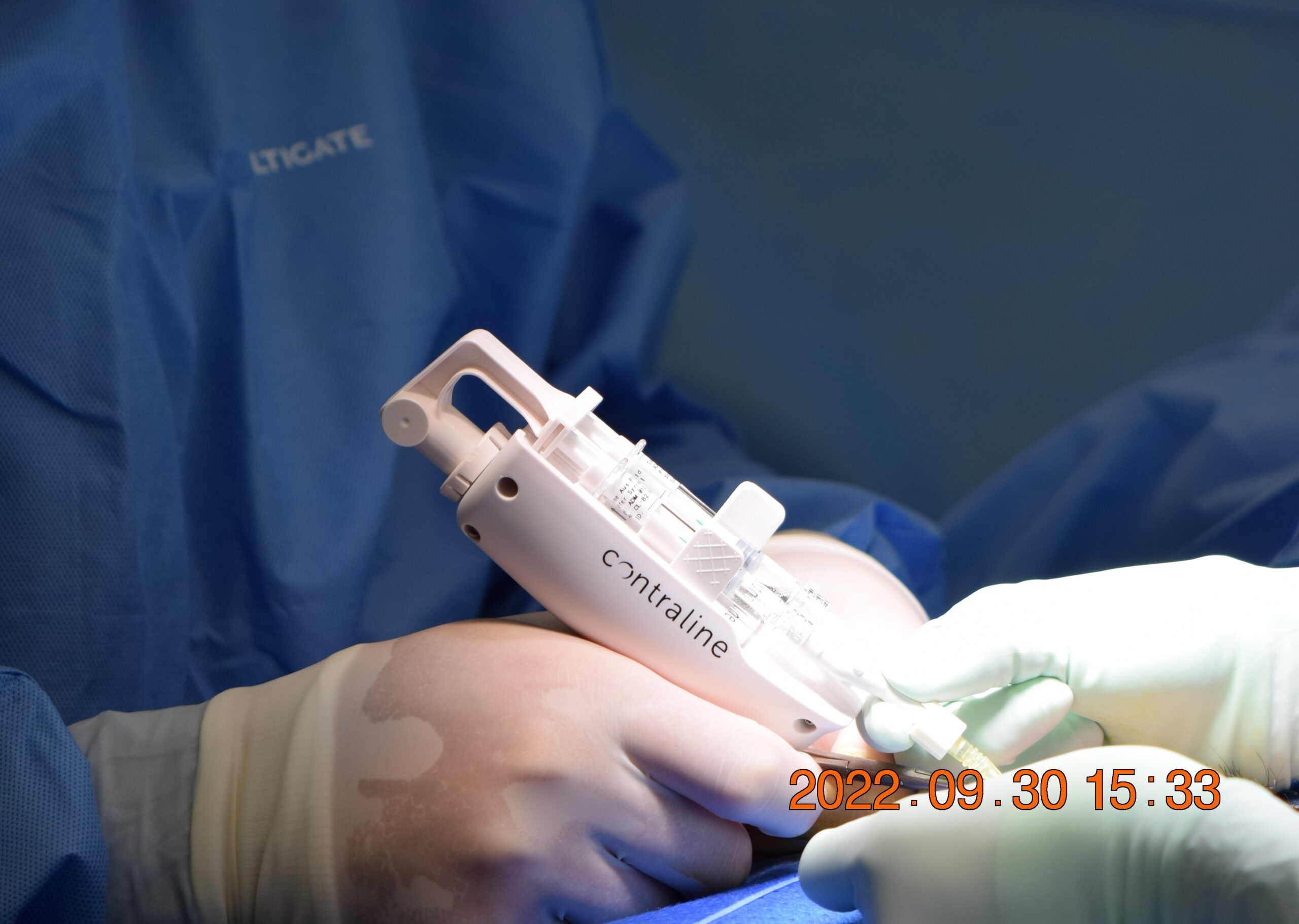
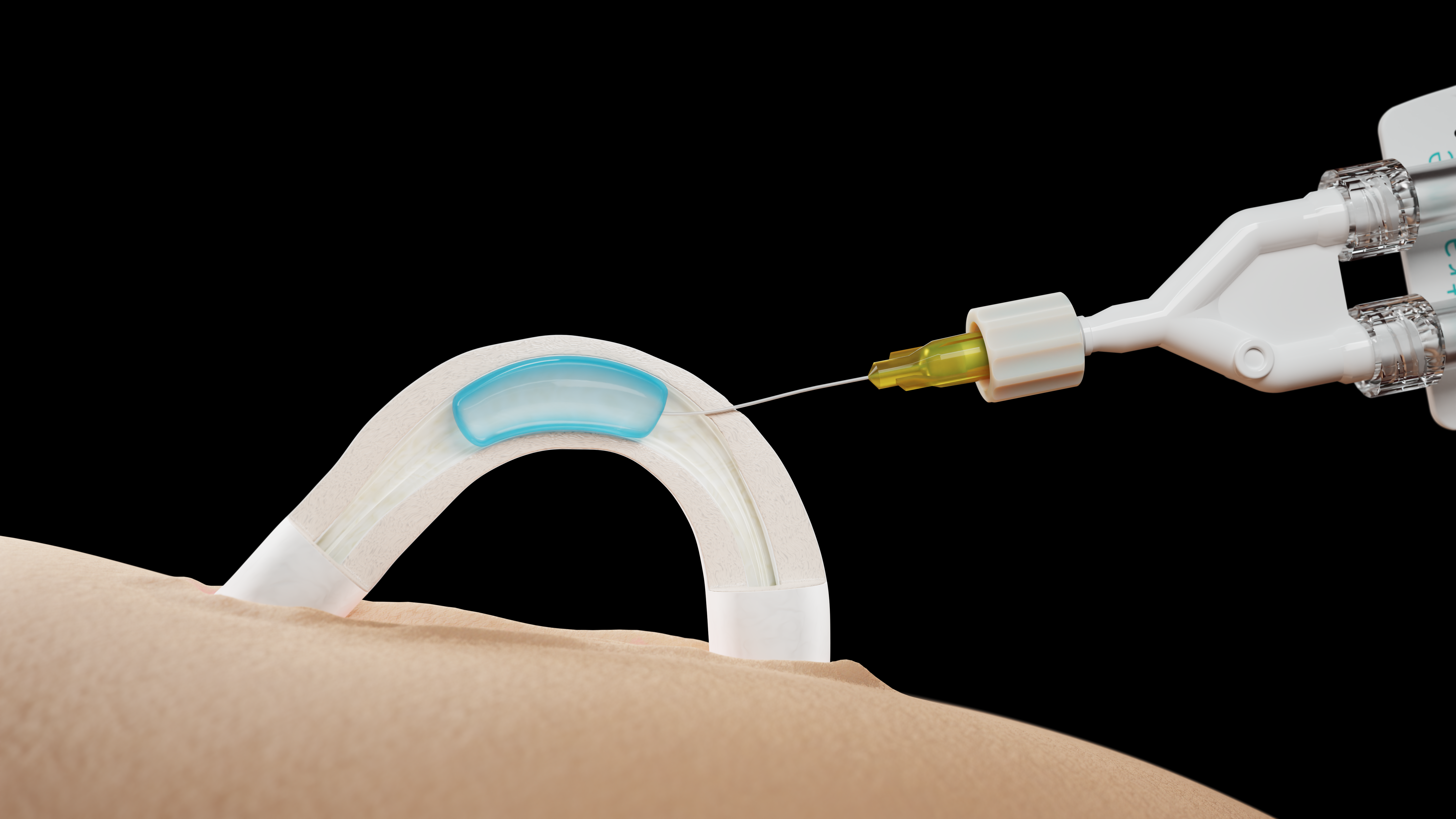
Medical device company Contraline became the first to implant a hydrogel-based male contraceptive gel into humans in a clinical trial. Implanted during a noninvasive 10-minute office procedure, the gel promises to block the flow of sperm to the vas deferens. If proven safe and effective, it could become the first nonhormonal, reversible contraceptive for men on the market.

How can specialty practices most effectively engage undertreated and underserved patients while navigating rising rates of workforce shortages, burnout and the financial pressures that come with operating a practice during a pandemic?

In a landscape where complexity has long been the norm, the power of one lies not just in unification, but in intelligence and automation.
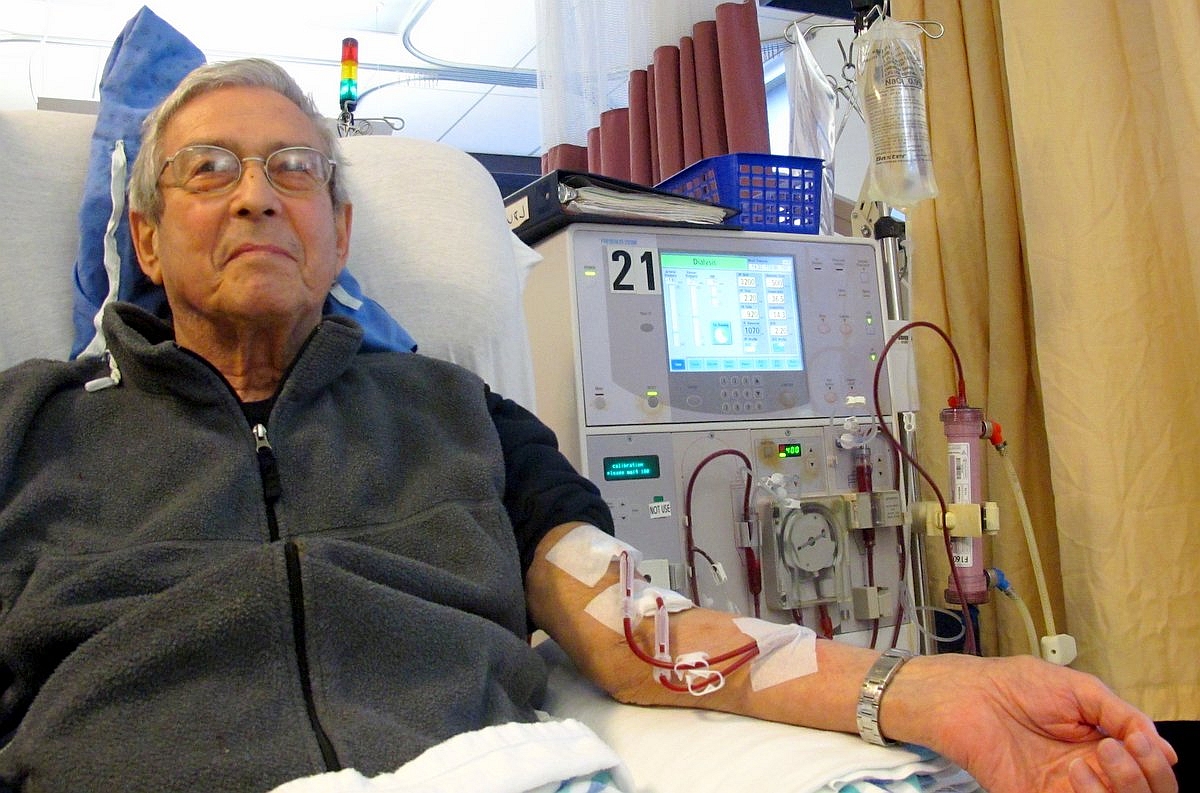
Home dialysis is not regarded as a threat to the viability of dialysis centers.

A surgical resident who was 11 years into his 15-year education to become a urologist called it quits the day before his 30th birthday. In addition to his investment of time to get this far, Sam Park has $100,000 in student loans. His story – told on an “Ask Me Anything” post on Reddit – […]

Market research has suggested that neuromodulation devices make up the fastest-growing segment of the medical device sector, forecasting some $7 billion in sales within five years. Although medical device giants Medtronic, St. Jude Medical and Boston Scientific dominate the market, there are a number of young companies working on nerve stimulation devices to treat pain […]

Botox is the miracle wrinkle-remover but if many were expecting that its approval to treat overactive bladder would cause wrinkles on the foreheads of senior management at Minnesota incontinence treatment company Uroplasty, they were mistaken. At least for now. In a conference call with analysts to discuss the company’s third fiscal quarter earnings Thursday, Uroplasty […]

The Food and Drug Administration has cleared an ultrasound bladder scanner made by Norwegian firm Vitacon AS to diagnose bladder dysfunction and the firm has signed a marketing agreement that will launch the product in the U.S. The VitaScan LT USB Scanner is an alternative to measuring bladder volume using urethral catheters, the company said […]

The global urology devices market is expected to be worth $8.6 billion by 2022, up from $4.4 billion in 2011, according to a report from London-based business intelligence firm Visiongain. The company projects that in 2012, the market will grow slightly to $4.6 billion. In 2011, the overall worldwide medical devices market was worth $266 […]

A New Zealand bladder cancer detection firm is opening its U.S. office at the biotechnology incubator Hershey Center for Applied Research, in a move that will add 100 jobs over three years. Pacific Edge Diagnostics will market its bladder cancer detection test, Cx Bladder, to urologists at the end of the year, according to a […]

Uromedica, which develops urinary incontinence treatments, will find out in as soon as a few weeks if its medical device for men is ready for U.S. Food and Drug Administration approval application. The Minnesota medical device company submitted fresh data to the FDA that, if accepted, would be the basis for a premarket approval (PMA) application, CEO Tim Cook said. Uromedica sent the data, which included a clinical analysis of more than 120 men, to the FDA in November. The company hopes to hear back from the agency by the end of January.

Medical device company US Endoscopy‘s urology division has released a new product, a biopsy and retrieval forceps device. The Mentor, Ohio-based company’s Alligator forceps provides the grip needed to safely and securely collect a biopsy or foreign bodies from the bladder or kidney, according to a statement from the company. Last year, the company formed […]